Solutions for Pharma Companies
Solutions for Pharma Companies
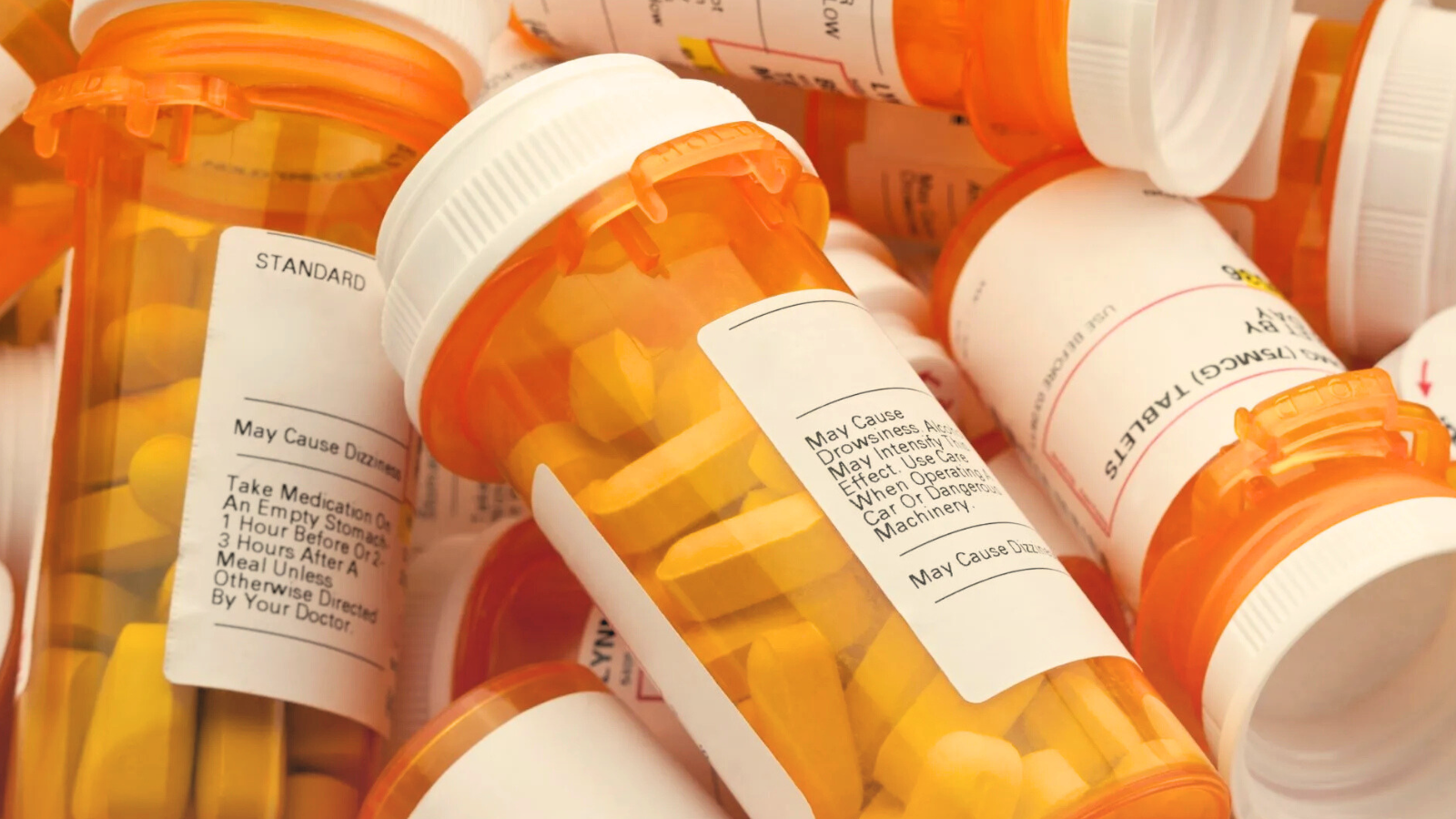
1. Drug Labels and Packaging Inserts
These contain essential information such as dosage instructions, warnings, storage guidelines, and expiration dates.
Also, we can provide translation services for Patient Information Leaflets (PILs).

1. Drug Labels and Packaging Inserts
These include appointment notices, billing statements, discharge summaries, and referral letters.
Translating these documents improves communication with patients, helps prevent missed appointments, and makes administrative tasks more efficient in multilingual settings.
2. Prescribing Information (PI) and Summary of Product Characteristics (SmPC)
These are detailed documents intended for healthcare professionals. They include pharmacological properties, contraindications, interactions, and administration methods.
Precise translation of PI and SmPC is required to prevent medical errors and meet regulatory standards.
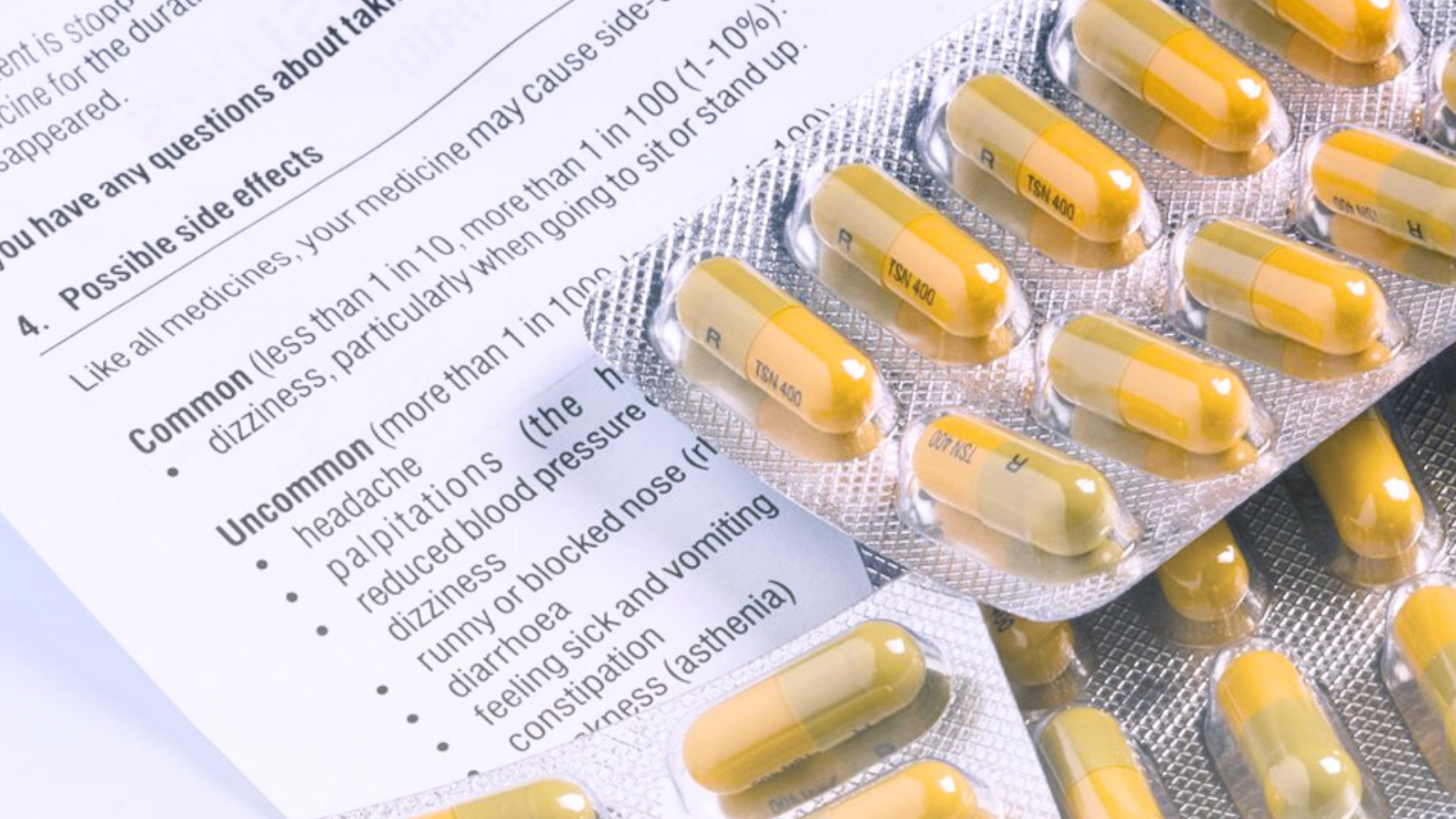

2. Prescribing Information (PI) and Summary of Product Characteristics (SmPC)
These are detailed documents intended for healthcare professionals. They include pharmacological properties, contraindications, interactions, and administration methods.
Precise translation of PI and SmPC is required to prevent medical errors and meet regulatory standards.

3. Regulatory Submissions
Examples of these include applications to health authorities such as the FDA or EMA, covering clinical trial data, manufacturing processes, and safety assessments.
Consistent and accurate translation is essential for approval processes across regions.

3. Regulatory Submissions
Examples of these include applications to health authorities such as the FDA or EMA, covering clinical trial data, manufacturing processes, and safety assessments.
Consistent and accurate translation is essential for approval processes across regions.
4. Clinical Study Protocols and Reports
This set of documents guide clinical trial operations and includes objectives, methodology, and research results.
Our translation services help all global sites follow the same procedures and report results in a consistent way. For pharmaceutical companies, this consistency is key to keeping data accurate, meeting regulatory standards, and speeding up the development of new treatments.


4. Clinical Study Protocols and Reports
This set of documents guide clinical trial operations and includes objectives, methodology, and research results.
Our translation services help all global sites follow the same procedures and report results in a consistent way. For pharmaceutical companies, this consistency is key to keeping data accurate, meeting regulatory standards, and speeding up the development of new treatments.

5. Marketing and Promotional Materials
These include brochures, advertisements, and digital content used to promote pharmaceutical products.
Translations must align with local language nuances while complying with advertising regulations.

5. Marketing and Promotional Materials
These include brochures, advertisements, and digital content used to promote pharmaceutical products.
Translations must align with local language nuances while complying with advertising regulations.
- Healthcare Translations © 2025 All Rights Reserved
Our translation and localization services designed for the diverse sectors of the healthcare industry.
Our collection of downloadable documents and articles that enhance our translation service offerings.
Request a quote with us. We respond in less than 24 hours.
- Healthcare Translations © 2025 All Rights Reserved

